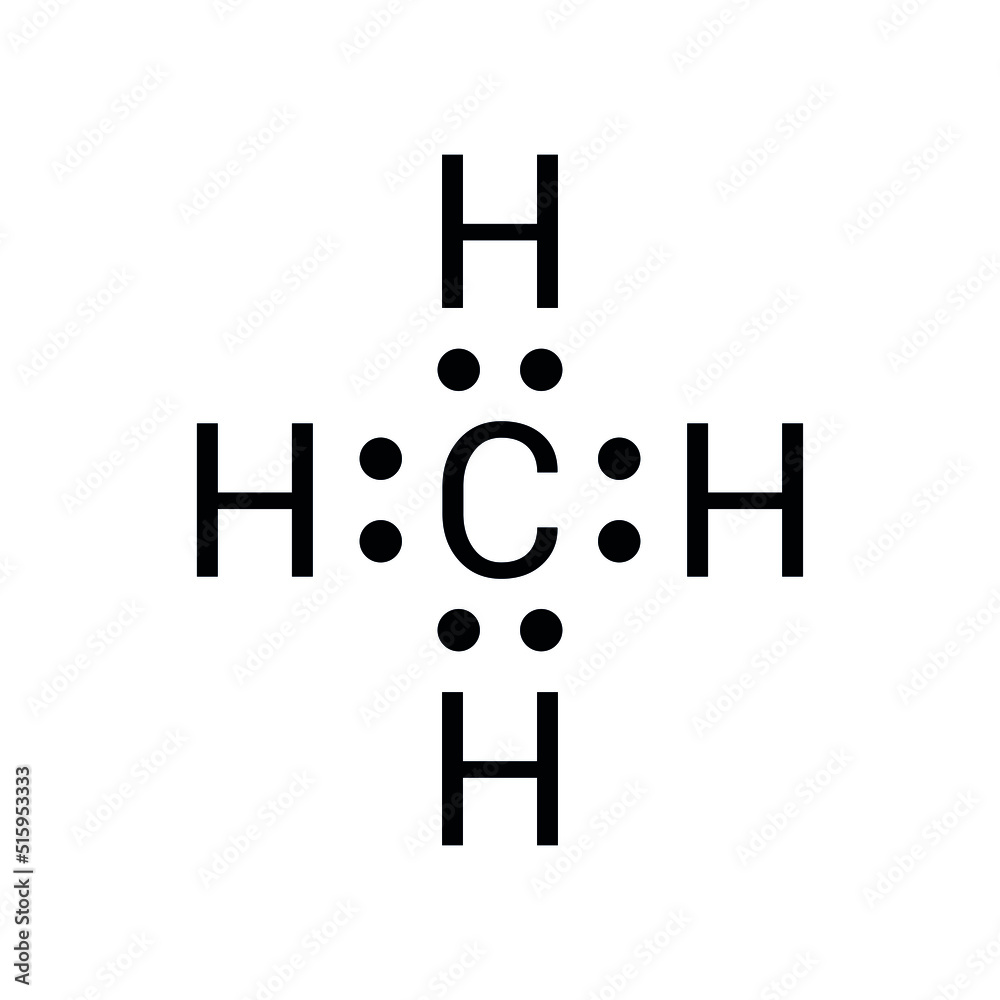
Ever wondered how the simplest molecule can unlock the secrets of the universe? Understanding the Methane Lewis Dot structure isn't just an academic exercise; it's the key to unraveling the complexities of molecular bonding and its impact on our world.
Methane, with its deceptively simple formula of CH₄, is a cornerstone of both organic chemistry and environmental science. The Lewis dot structure offers a clear visual representation of the bonds between the carbon atom and its four hydrogen partners, allowing us to grasp the molecule's behavior in diverse settings.
This exploration will dissect the Methane Lewis Dot structure, illuminating its practical applications and highlighting its importance in the broader field of chemistry. Whether you are a seasoned chemist or a curious student, this guide offers everything necessary to fully understand methane's electron distribution and molecular architecture.
- Jameliz Benitez Smith A Comprehensive Look Into Her Life And Career
- Marie Temara Nudes Debunking Myths And Exploring The Truth
| Aspect | Details |
|---|---|
| Molecular Formula | CH₄ |
| Central Atom | Carbon (C) |
| Number of Valence Electrons | 8 (4 from Carbon, 1 from each Hydrogen) |
| Type of Bonds | 4 Single Covalent Bonds (C-H) |
| Molecular Geometry | Tetrahedral |
| Bond Angle | Approximately 109.5 degrees |
| Hybridization | sp3 |
| Common Uses | Fuel (Natural Gas), Production of chemicals, Energy source |
| Environmental Concern | Greenhouse Gas, Contributor to Global Warming |
| Reference Website | LibreTexts Chemistry |
- Aishah Sofey Leaks Unveiling The Truth Behind The Controversy
- Masa49 Exploring The Unique Aspects And Importance Of The Masa49 Concept